آقاي ساسان سلام
اگر بيماري در حال پيشرفت باشد،نقاط جديد بدن كه تازه دارند رنگدانه از دست مي دهند ولي هنوز روند پيشرفت ادامه دارد،خيلي سفيد نيستند،در خصوص سوال دوم،چنين آمپول و درماني وجود ندارد،بهترين درمان براي شما نور درماني هست .
Photodynamic therapy (PDT) aims to destroy the desired target selectively, thereby minimizing damage to normal tissue.
The photodynamic reaction consists of the excitation of photosensitizers (usually porphyrins) by visible light in the presence of oxygen, resulting in the generation of reactive oxygen species, particularly singlet oxygen.
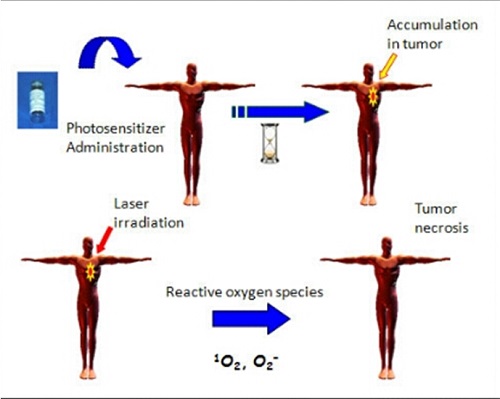
These reactive oxygen species mediate cellular and vascular effects, depending on the tissue localization of the photosensitizer, and results in a direct or indirect cytotoxic effect on the target cells.
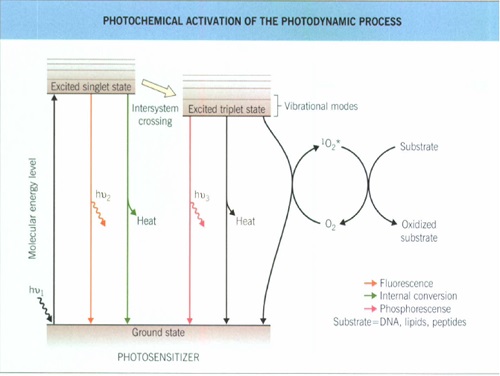
The biologic effects can be divided into primary cellular and secondary vascular damage
Depending on the intracellulaIr localization of the photosensitizers damage to sub-cellular structures, such as mitochondria, lysosomes, or endoplasmic reticulum, also occurs, whereas DNA is not a primary target.
These direct effects probably play a key role in topical PDT whereas vascular effects after systemic administration of photosensitizers appear to be the decisive event.
These effects consist of vasoconstriction, blood stasis, and thrombosis of tumor vessels leading to tumor ischemia and subsequent necrosis.
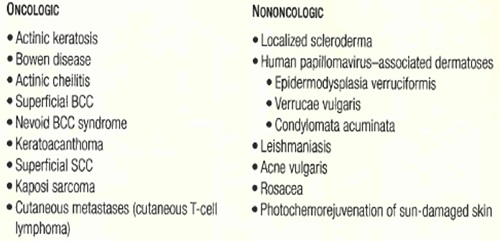
In dermatology, PDT has been used effectively for pre-cancerous and malignant conditions such as actinic keratosis, BCC, Bowen disease, and superficial squamous cell carcinoma, as well as for inflammatory dermatoses such as localized scleroderma, acne vulgaris, and leishmaniasis.
PHOTOSENSITIZETR:
ideal photo sensitizer for PDT in dermatology should meet the following criteria:
(1) chemical purity
(2) high singlet-oxygen quantum yield,
(3) significant light absorption at wavelengths that penetrate the skin sufficiently deeply,
(4) high tissue selectiviry
(5) efficacy after topical application
Δ-aminolevulinic acid (ALA) or its methyl ester (MAL), intermediates in heme biosynthesis, induces the synthesis of photosensitizing protoporphyrin IX in the target tissue.
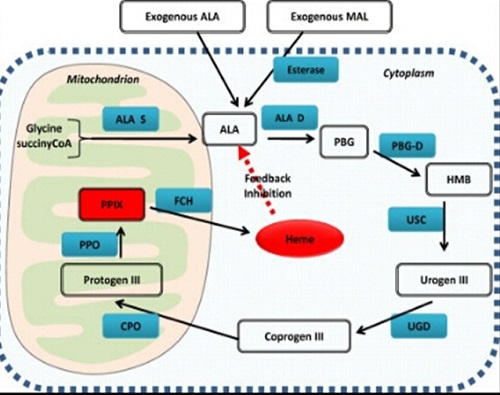
In this case, the concentration of the photosensitizer depends on the metabolic status of the diseased tissue.
Most photosensitizers generate singlet oxygen with a quantum yield between 5 percent and 20 percent.
A high quantum yield means that less sensitizer is required in the target tissue to induce sufficient PDT effects.
The light absorption maxima of the current sensitizers is in the visible range (400 nm to 700 nm).
In this range, light penetration in tissue is only up to 3 mm, limiting PDT to superficial tumors unless interstitial light propagation is used .
A high selectivity for sensitizer accumulation in target tissue is necessary to avoid damage to surrounding normal tissue, and this is particularly important when larger areas are treated (e.g., actinic keratoses).
So far, only AIA or MAI show reasonably high selectivity after topical application.
The ratio of porphyrin induction in skin tumors to the surrounding tissue is higher than 10:1 likely due to a combination of enhanced AlA-MAL penetration through an abnormal stratum corneum and altered metabolism and accumulation within the pre-malignant or malignant cells.
Light penetration into skin increases with longer wavelengths up to 1100 nm. Although porphyrins absorb maximally in the Soret band (400 to 410 nm, blue light), there are minor absorption peaks at longer visible light wavelengths.
To increase depth of penetration while matching the absorption maxima of porphyrin photosensitizers, wavelengths around 630 nm are often used.
Lasers are effective but they are quite expensive, require regular maintenance, and have a small treatment aperture
Hence, simpler incoherent light sources represent a valuable alternative.
fluorescent lamps or light-emitting diodes with appropriate red or blue light emission are commercially available and designed for treatment of large surface areas.
Also, intense pulsed light sources are used for dermatologic PDT
Dosimetry depends on the photosensitizer and light source used, as well as on the condition to be treated.
For PDT of epithelial cancer, photosensitization must be sufficient to induce necrosis or apoptosis.
for treating inflammatory dermatoses, significantly lower doses suffice because the goal appears not to be cell death but rather sublethal damage or modulation of cellular functions.
the skin can be sensitized by either intravenous, topical, or intralesional routes of administration of the photosensitizer.

Small hydrophilic molecules like AIA or MAL penetrate well into the skin, particularly if the stratum corneum is abnormal, as is the case in some epidermal tumors.
In addition, epidermal cells and the pilosebaceous unit synthesize porphyrins to a much greater extent than fibroblasts,myocytes,or endothelialc ells
Epithelial tumors generally synthesize much higher amounts of protoporphyrin IX than the surrounding tissue and can therefore be destroyed without equivalent damage to healthy skin
Topical AIA-Mal-induced photosensitivity is thus restricted to the target area.
Systemic porphyrin induction is not observed after topical application. The only significant side effect of topical AIA-MAI PDT is a stinging pain during and shortly after irradiation, proportional to the intensity of the phototoxicity reaction.
Bowen & SCC
Systemic PDT with porfimer sodium for Bowen disease is very effective , but invasive squamous cell carcinomas respond less well, with recurrence rates of up to 50 percent within 6 months.
AK
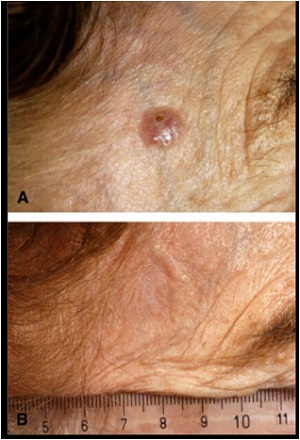
Up to 90% of AKs can be cleared with topical PDT.
Hypertrophic AKs may prove more difficult to eradicate due to drug and light penetration issues; so, prior to PDT, curettage debridement of scale is often recommended.
AKs can be treated on a lesion-by-lesion basis, or by applying photosensitizer to a broad area of photodamaged skin harboring active lesions.
This approach allows for concomitant treatment of subclinical Aks.
Actinic cheilitis represents an excellent potential PDT application since alternative treatment approaches such as surgical vermilionectomy or laser vaporization are more complicated and morbid.
BCC
Superficial BCCs respond well to PDT, with an expected primary clearance rate of 87% with a follow-up of 3-36 months.
Nodular BCCs do not appear to be as well suited to PDT as a lower response rate of 53-71 % has been observed.
Multiple BCCs arising in the context of cancer-prone genodermatoses such as nevoid basal cell carcinoma syndrome are probably a good application for PDT since multiple lesions can be treated during one session.
Psoriasis
In theory and practice, psoriatic plaques can be readily photosensitized by topical agents used in PDT.
Lesional clearing has been observed with various light sources, including UVA and red light. High levels of PplX within treated plaques correlated with significant and often dramatic pain upon light exposure.
Pain, combined with the need for multiple treatments and a controlled trial demonstrating results inferior to narrowband UVB phototherapy have made PDT a much less attractive option for psoriasis when compared to existing therapies, including targeted molecular therapies (biologics).
However, cutaneous metastases of malignant melanoma, pigmented BCC, and sclerodermiform variants of BCC respond poorly to AIA PDT probably because of insufficient porphyrin synthesis and/ or penetration of light within the lesions. treatment efficacy is enhanced by repeated treatment sessions.
Tcell lymphoma
Activated and malignant T cells can be selectively sensitized by exogenous ALA, and this is why PDT has been used to treat the mycosis fungoides form of cutaneous T-cell lymphoma.
Interestingly, these lymphocytes express high levels of transferrin and have a corresponding low intracellular level of iron, conditions that favor PpIX accumulation
Because sufficient topical ALA can reach the target T cells within the epidermis and dermis, an appreciable clinical effect can be observed.
acne
it should be noted that propionibacterium acnes produces cutaneous porphyrins, which can be readily demonstrated by Wood's light examination of any sebaceous-dense area, including normal skin.
Exposure to high-intensity blue light alone, in the absence of any exogenous PDT drug, can lead to clinical improvement of up to two-thirds of inflammatory acne lesions after eight treatment sessions.
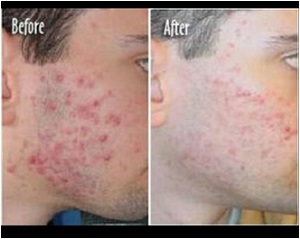
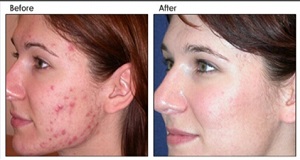
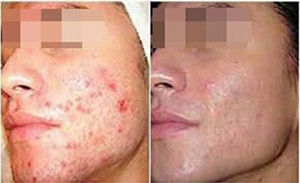
photorejuvenation
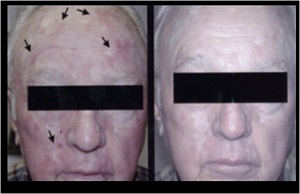
PDT with ALA has also demonstrated to enhance the treatment of photodamaged skin with a variety of lasers and light sources. Improvement of global appearance, fine lines, tactile skin roughness, mottled hyperpigmentation, and telangiectasias has been described.
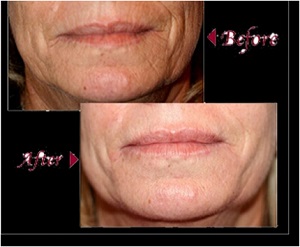
infection
There arc several mechanisms by which PDT can treat infectious diseases.
Singlet oxygen may destroy the microbes directly or it may activate host immunologic reactions against the microorganisms.
In addition, PDT can induce necrosis of infected tissue, for example, in the treatment of verrucae or condylomata.
Conditions that have been shown in controlled trials to improve with PDT include verruca vulgaris, condyloma acuminata and leishmaniasis
The emergence of drug-resistant bacteria has also motivated investigations into the treatment of bacterial skin infections with PDT.
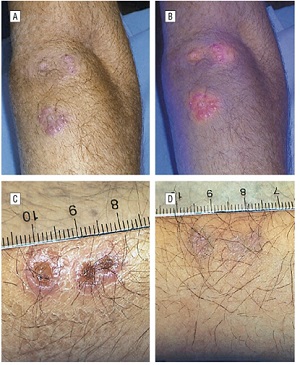
Vascular lesions
Systemic photosensitizers are biodistributed via the peripheral circulation and it stands to reason that intravascular drug activation with light would be useful in inducing the involution of abnormal vascular structures.
Nowadays, this effect is clearly operative in two clinical situations:
1) vascular bed damage and ischemic necrosis following systemic photosensitization of carcinomas and
2) destruction of hyperproliferative retinal blood vessels in age-related macular degeneration with systemic verteporfin-based PDT.
Vascular malformations such as port-wine stains have been shown to respond to systemic PDT with results that appear to be at least equivalent to those achieved with conventional pulsed laser therapy.
Adverse effects
adverse effects related to PDT are largely an extension of the photochemical and photobiologic reactions that involve the generation of reactive oxygen moieties, especially singlet oxygen.
Interestingly, erythropoietic protoporphyria could be considered a possible toxicity model for ALA, since both clinical scenarios result in the overproduction of PpIX.
The primary difference is that with erythropoietic protoporphyria, PpIX excess is constitutive, whereas with ALA-based PDT, the effect is transient.
Systemic photosensitizers carry a greater risk of photosensitization and the risk depends on the particular agent and its dose.
Photosensitivity manifests as varying degrees of erythema, edema and/or blistering of any exposed site, with subjective symptoms of pain, a burning sensation, lightheadedness and photophobia.
Patients receiving systemic PDT photosensitizers must be instructed to strictly avoid outdoor light as well as bright indoor light. Appropriate opaque clothing must be worn.
Conventional chemical sunscreens, even those with a high sun protection factor, are not useful in managing at-risk patients since the action spectrum extends throughout the visible spectrum.
Complete avoidance of all light is unnecessary, since regular indoor light exposure provides a safe means by which low-level photobleaching can hasten the clearance of photoactive agents from the skin.
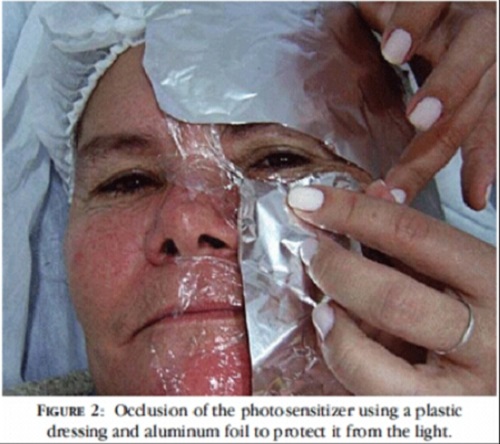
In the case of topical PDT, applying an opaque covering to the treatment site during the incubation period will prevent inadvertent activation.
Within seconds to minutes of illumination of the skin with light, patients will often experience a range and series of unpleasant sensations at the site of light exposure ; pain, warmth, burning, stinging, smarting and stabbing.
Inflammation:
At the end of the light exposure, the treated site will exhibit an immediate wheal and flare reaction.
Over the next 24-48 hours, there may be edema, induration, purpura and blistering, along with exudation and crusting.
Depending on the indication and the PDT drug-light dose, frank tissue necrosis may supervene accompanied by erosions and ulceration.
Local reactions evolve over the ensuing 1-6 weeks and any wounds will heal by second intention.
As with any physical modality, PDT can induce scarring and postinflammatory hyper- and hypopigmentation.
Development of these complications depends primarily on the extent of the iatrogenic injury and the patient's constitutive injury and wound healing propensity.
Multiple case reports have documented contact hypersensitivity to mALA.
آقاي ساسان سلام
اگر بيماري در حال پيشرفت باشد،نقاط جديد بدن كه تازه دارند رنگدانه از دست مي دهند ولي هنوز روند پيشرفت ادامه دارد،خيلي سفيد نيستند،در خصوص سوال دوم،چنين آمپول و درماني وجود ندارد،بهترين درمان براي شما نور درماني هست .
زمان بهترین و ارزشمندترین هدیه ای است كه می توان به كسی ارزانی داشت.هنگامی كه برای كسی وقت می گذاریم، قسمتی از زندگی خود را به او میدهیم كه باز پس گرفته نمی شود . باعث خوشحالی و افتخار من است كه برای عزیزی مثل شما وقت می گذارم و امیدوارم كه با راهنماییهای اساتید این رشته واظهار نظر شما عزیزان این سایت آموزشی پر بارتر گردد.