آقاي ساسان سلام
اگر بيماري در حال پيشرفت باشد،نقاط جديد بدن كه تازه دارند رنگدانه از دست مي دهند ولي هنوز روند پيشرفت ادامه دارد،خيلي سفيد نيستند،در خصوص سوال دوم،چنين آمپول و درماني وجود ندارد،بهترين درمان براي شما نور درماني هست .
Journal of the American Academy of Dermatology
September 2011 / Volume 65 / Number 3
Jesse M. Lewin, MD, Jeremy A. Brauer, MD, and Ariel Ostad, MD New York, New York
The use of electrosurgery and lasers by dermatologists and dermatologic surgeons has increased in recent years with the growth of technology and procedures performed. These devices produce surgical smoke that has been demonstrated to harbor live viruses and bacteria in addition to hazardous chemicals.
According to theAmerican Society of Plastic Surgeons, in 2008 there were 400,262 laser skin resurfacing procedures performed in the United States, a 134% increase from 2000.
In addition, the proportion of dermatology visits with associated procedures has trended upward, with procedures performed at 29.8% of the visits in 1995 and 40.1% of the visits in 2001.
In this time period the most frequent procedures performed were excision and destruction, including electrodessication.
There is mounting evidence that the gaseous products from lasers contain DNA from viruses including HIV and HPV.
In addition to the potential for contracting infectious diseases from surgical smoke, smoke collected during surgery using both electrocautery and laser has been shown to be mutagenic.
Lastly, studies also demonstrate that those exposed to surgical smoke may develop allergic sensitization to these bio-aerosols With an increase in the number and frequency of procedures performed by dermatologists using electrocautery and lasers, a review of the contents and effects of gaseous byproducts or ‘‘surgical smoke’’ produced by these devices is crucial.
This is a topic that has been described in the surgical literature but there is a paucity of information in the dermatologic literature and it deserves attention to protect dermatologists, our staff, and our patients.
In addition, formal guidelines dictating appropriate protection against the effects of surgical smoke are designed for the operating room rather than the outpatient office setting where most dermatologic procedures are performed.
Surgical smoke is created when electrocautery and lasers heat target cells to the point of boiling leading to membrane rupture and the dispersal of cellular contents as fine particles.
The resultant surgical smoke is composed of 95% water and 5% particulate matter, which is composed of chemicals, blood and tissue particles, viruses, and bacteria.
The size of the particulate matter is dictated by the device used, with electrosurgical units creating particles of roughly 0.07 µm and lasers liberating particles of 0.31 µm.
The size of liberated particles is important as it is known that those smaller than 100µm in diameter remain airborne and particles less than 2µm are deposited in the bronchioles and alveoli.
Traditional surgical masks are able to capture particles greater than 5 µm but offer no protection against particulate matter produced by electrosurgical and laser devices liberating byproducts less than 1 µm.
The chemical contents of surgical smoke have been well described in the literature. Electrocautery has been shown to produce a plume composed mostly of hydrocarbons, phenols, nitriles, and fatty acids.
In addition, carbon monoxide,acrylonitrile, and hydrogen cyanide are liberated and receive the most attention regarding their harmful effects.
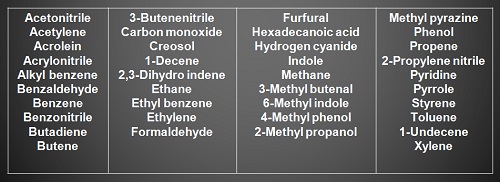
Acrylonitrile, a pungent-smelling colorless liquid that forms hydrogen cyanide is classified as a class 2A carcinogen, which is probably carcinogenic to human beings, and is absorbed through the skin and lungs.
Short-term exposure to acrylonitrile is known to cause eye irritation, nausea, vomiting, headache, sneezing, weakness, and lightheadedness, whereas long-term exposure causes cancer in animals and has been linked to a higher incidence of cancer in human beings.
The level of hydrogen cyanide in surgical smoke has been shown to be 30 times less than that of directly inhaled smoke from a cigarette, whereas the level of butadiene is 100 times less.
However, the nature of these compounds warrants concern as it has been suggested that butadiene
accounts for 45% of the cancer potency and hydrogen cyanide 89% of the cardiovascular potency in cigarette smoke.
Based on their findings, the authors concluded that the organic compounds in surgical smoke may represent a health hazard similar to chronic second-hand smoke exposure, a point that should not be ignored by dermatologists who are routinely exposed during their daily practice.
Another component of surgical smoke known to have serious health risks is benzene.
According to the Occupational and Safety Health Administration (OSHA), the short-term effects of benzene include headache; dizziness; nausea; and irritation of the eyes, nose, and respiratory tracts.
As stated by OSHA, chronic exposure to benzene even at low concentrations may result in blood disorders ranging from anemia to leukemia.
A permissible exposure limit of 10 ppm was established by OSHA.
In a study by Sagar et al in 1996, benzene concentration of 0.02 ppm was extracted from surgical smoke obtained during colorectal surgery.
It is difficult to extrapolate the concentration of hydrocarbons such as benzene, produced during cutaneous surgery as performed routinely by dermatologists.
In addition, it is unclear what effect the chronicity of low-level exposure has on human physiology.
It is important to have an understanding of the chemical contents of surgical smoke, and respect for the unanswered questions surrounding long-term and acute effects of exposure.
One of the first insights into the mutagenicity of surgical smoke was the demonstration that electrocauterization performed on the mucosa of the canine tongue lead to mutagenic activity on a Salmonella TA98 tester strain.
The authors concluded that the mutagenic potency observed with electrocautery smoke was comparable with that of cigarette smoke.
They quantified that the mutagenic effect of these smoke condensates from 1 g of cauterized tissue with laser and electrocautery was equivalent to those from 3 or 6 cigarettes, respectively.
Human tissue has also been used to demonstrate the mutagenic potential of electrocautery surgical smoke by collecting samples during reduction mammoplasty.
The authors collected air samples from the operating department during two such procedures and used two tester strains of Salmonella, TA98 and TA100.
Just as in the aforementioned study, this study demonstrated that a Salmonella TA98 strain underwent alteration of its histidine dependence when exposed to the smoke extract from human tissue ablation.
Although it is unclear whether the mutagenicity of surgical smoke poses a direct risk to human beings, and if so if there is a minimal ‘‘dose,’’ these cytogenetic data suggest that further investigation and tighter precautions are warranted.
In addition to the ability to mutate DNA, surgical smoke contains transmissible, viable malignant cells.
In one study, pellets of B16-F0 mouse melanoma cells were cauterized and cell viability in surgical smoke assessed via a trypan blue assay, immediately after collection and 7 days later.
In addition, a tetrazolium viability assay was performed to assess cell viability after cauterization of tumor pellets at 10, 20, and 30 W for 5 seconds.
Intact viable malignant cells were present in the surgical smoke immediately after collection and after 5-second bursts of cautery at various wattages.
In addition, lower cautery levels were associated with higher mean concentrations of viable melanoma cells.
Although to our knowledge no reported cases of resultant malignant transformation in a human host exist, the aerosolization of malignant cells identified in this in vitro study underscores the importance of respiratory precautions and smoke evacuation during the surgical treatment of cutaneous malignancies including melanoma, basal cell carcinoma, and squamous cell carcinoma by dermatologists.
Numerous studies have demonstrated the adverse effects of surgical smoke on the respiratory system in animal models.
In one study, 12 rats were exposed to the surgical plume produced by ablation of pig skin with electrocautery and a ND:YAG laser.
After humane euthanasia the authors examined the animals’ lung parenchyma and observed the development of blood vessel hypertrophy, alveolar congestion, and emphysematous changes.
These changes were less severe in rats exposed to surgical smoke collected with single-and double-filtered smoke evacuators than unfiltered smoke.
The authors postulated that benzene, formaldehyde, and acrolein extracted from the smoke may have been responsible for the observed pulmonary damage.
A similar study examined the effects of longterm inhalation of carbon dioxide (CO2) laser smoke on the lungs of rats . It was determined that particulate matter produced by tissue vaporization was deposited in the alveoli of the animals and produced pathologic changes consistent with interstitial pneumonia, bronchiolitis, and emphysema.
Importantly, the severity of these changes increased proportionately with the duration of exposure.
Both studies advocated for the use of smoke evacuators as a protective measure.
The number of studies demonstrating the infectious nature of surgical smoke has grown considerably.
To date, the focus of the literature on this subject has centered on the viability of viruses in electrocautery and laser plumes.
As early as 1988, Garden et al recovered intact bovine papillomavirus and HPV from the plume of CO2 laser-treated human and bovine lesions. In the next few years two clinical surveys of laser users revealed increased infections with HPV.
In 1995, Gloster and Roenigk at the Mayo Clinic conducted a comparative study using questionnaires sent to members of the American Society for Laser Surgeons and the American Society of Dermatologic Surgery.
The comparison groups were CO2 laser surgeons and two large groups of patients in the community with a diagnosis of warts. Analysis revealed that CO2 laser surgeons had a statistically significant greater risk of acquiring nasopharyngeal lesions but were less likely to acquire plantar, genital, and perianal warts than the Mayo Clinic patient group.
The authors presumed that inhalation of the laser plume is a likely means by which HPV can be transmitted to the upper airway, suggesting that those using lasers to treat HPV lesions are at greater risk.
In 2002, Garden et al demonstrated for the first time that surgical smoke can reproduce disease. CO2 laser aerosol was collected from tissue infected with bovine papillomavirus using various laser settings and inoculated calves with the surgical plume.
The authors found that through inoculation, these animals developed tumors that were proven biochemically and histologically to be infected with the same virus type as was present in the laser plume.
Although direct inoculation with bovine papillomavirus laser plume may not compare with clinical exposure during procedures, a review of the literature identified two reports of health care providers developing laryngeal papillomatosis as a result of laser and electrodessication of HPV-related anogenital condyloma with CO2 and neodymium:yttrium-aluminumgarnet lasers , in addition to the earlier study by Gloster and Roenigk discussed above.
In addition to the potential for viral aerosolization with laser and electrosurgical plume, viable bacteria
have been detected during laser resurfacing, a procedure widely practiced by dermatologists and
plastic surgeons today.
Specimens from 13 procedures were collected, and cultures grew coagulasenegative Staphylococcus, Corynebacterium, and Neisseria.
The use of smoke evacuation systems to prevent the transmission of these pathogens to those performing laser resurfacing was recommended by the authors.
Although guidelines exist for protection against surgical smoke, it is the experience of the authors that many dermatologists in practice use minimal or no precautions while using electrocautery or lasers.
A recent World Wide Webebased survey study examined current surgical smoke practices in North America and Canada.
A section on surgical smoke control practices was divided into local exhaust ventilation (LEV) procedures, which included wall suction or smoke evacuator, and respiratory protection, which included surgical mask, laser mask, N-95, or other National Institute for Occupational Safety and Health (NIOSH)-approved respirator.
There were 623 respondents to the survey, which included individuals from all 50 states and Canada. Of respondents,
86% were perioperative nurses, with 56% participating in cosmetic/plastic surgery and 6% in dermatology.
The researchers found that LEV is used by fewer than half of the facilities represented by survey respondents for most laser procedures and in very few facilities for most electrosurgery, electrocautery, or diathermy procedures.
In addition, the collected data suggested an alarmingly low use of respiratory protection equipment. Of particular interest to dermatologists, the percentage of survey respondents answering that smoke evacuators were used ‘‘never or seldom’’ for benign skin lesion removal and malignant skin lesion removal using electrosurgery was 82% and 83%, respectively. Upon answering the same question for the use of lasers to remove benign and malignant skin lesions, 65% and 61% of respondents responded ‘‘never or seldom,’’ respectively.
In terms of respiratory protection, only 5% of respondents admitted tousing an N-95 or NIOSH-approved respirator ‘‘always or often’’ during benign skin lesion removal with both electrocautery and laser.
It is clear from this study that, despite recommendations from various professional organizations advocating the use of LEV and respiratory precautions, these measures are not being widely used.
Of the 132 respondents who wrote comments in the survey, 58 specified obstacles to compliance with smoke control practices.
Interestingly, 32 of these 58 reported that surgeon resistance or refusal to allow LEV use was an obstacle to compliance.
As physicians we must realize that our decision not to use protective measures against surgical smoke puts not only ourselves but our staff at risk.
It is evident from our review of the literature that surgical smoke poses potential health risks to dermatologists who perform procedures using electrocautery and lasers. Although it is impossible to calculate this risk, the studies reviewed indicate the potential for infection, carcinogenesis, and pulmonary damage as a result of exposure to surgical plume.
The majority of authors and the aforementioned guidelines recommend the use of smoke evacuation and surgical masks, however, these measures are not consistently implemented, nor are they legally mandated.
Although surgical masks have varied reported efficacies ranging from protection against particles as small as 1 µm to allowing passage of those sized 9 µm, it is clear that their use is important but not sufficient.
Laser masks or highfiltration masks, provide greater protection than standard surgical masks and are able to filter particles to 1.1 µm; however, it has been shown that approximately 77% of particulate matter in surgical smoke is 1.1 µm and smaller.
In addition to the use of masks, many believe that the most important protective measure against surgical smoke is consistent and correct use of smoke evacuation.
It has been shown that smoke evacuators are 98.6% effective when placed 1 cm from the treatment site, with efficacy decreasing to 50% when moved to 2 cm from the treatment site.
One study demonstrated that suction clearance of surgical smoke with a smoke evacuator resulted in a significant reduction in the amount of smoke reaching the level of the operator’s mask during thyroid surgery.
Therefore we recommend diligent use of highfiltration masks in addition to smoke evacuation systems to dermatologists performing laser surgery and using electrocautery.
We also advocate further research into quantifying the exposure of dermatologists to surgical smoke in the outpatient setting.
Lastly, a qualitative and quantitative study examining current practices in the dermatology community and barriers to smoke protection implementation may also provide an impetus for further education.
آقاي ساسان سلام
اگر بيماري در حال پيشرفت باشد،نقاط جديد بدن كه تازه دارند رنگدانه از دست مي دهند ولي هنوز روند پيشرفت ادامه دارد،خيلي سفيد نيستند،در خصوص سوال دوم،چنين آمپول و درماني وجود ندارد،بهترين درمان براي شما نور درماني هست .
زمان بهترین و ارزشمندترین هدیه ای است كه می توان به كسی ارزانی داشت.هنگامی كه برای كسی وقت می گذاریم، قسمتی از زندگی خود را به او میدهیم كه باز پس گرفته نمی شود . باعث خوشحالی و افتخار من است كه برای عزیزی مثل شما وقت می گذارم و امیدوارم كه با راهنماییهای اساتید این رشته واظهار نظر شما عزیزان این سایت آموزشی پر بارتر گردد.