آقاي ساسان سلام
اگر بيماري در حال پيشرفت باشد،نقاط جديد بدن كه تازه دارند رنگدانه از دست مي دهند ولي هنوز روند پيشرفت ادامه دارد،خيلي سفيد نيستند،در خصوص سوال دوم،چنين آمپول و درماني وجود ندارد،بهترين درمان براي شما نور درماني هست .
THE FUNCTIONS OF A WOUND DRESSING
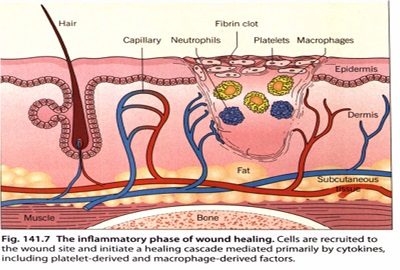
A wound dressing is a substitute for native epithelium lost as a result of injury. The process of wound repair occurs in stages each of which may require different dressings.
The main function of a dressing is to provide the optimum environment for rapid healing by protecting the wound from further trauma, bacterial invasion or exposure to caustic substances, which is especially important during the early acute, inflammatory stage.
The dressing should ideally be able to
conform to the wound shape,
absorb wound fluid without increasing
bacterial proliferation or causing excessive desiccation, pressure for hemostasis, and prevent leakage from the bandage.
Eliminate pain,
promote re-epithelialization during the reparative phase, and be easily applied and removed with minimal injury to the wound.
does not shed fibers or compounds into the wound that could lead to a foreign body reaction or an irritant or allergic reactions.
مقرون به صرفه باشد.
بررسی های زیادی در مورد به صرفه بودن پانسمانها انجام شده كه نشان داده اند استفاده از محصولات درمان زخم مدرن و گران می توانند از روش سنتی و مواد مصرفی ارزان،مقرون به صرفه تر باشد.طول دوره درمان و تعداد تعویض در این میان نقش موثرتری دارند.
درمان سریعتر،كاهش طول مدت بستری،لوازم مصرفی كمتر،عوارض كمتر و صرف وقت كمتر توسط پرستاران و پزشكان، توجیه كننده مقرون به صرفه بودن و كاهش هزینه های درمان با این نوع پانسمانها هستند. مشاهده و بررسی زخم با وجود آنها ساده باشد.
خواص و ویژگیهای خود را بطور ثابت حفظ كند.یعنی در اثر تغییرات دما و رطوبت خواص آن تغییر نكند.
قابل احتراق نباشد.
این امر خصوصا در بیمارنی كه زخم پا دارند و نزدیك به منبع حرارت می نشینند مهم است.
قابل استریل شدن باشد.
پانسمان باید شكل انعطاف پذیر و قابل انعطاف بوده و برای استفاده در سطوح ناهموار بدن،شكل مناسب داشته باشد.
باید در دسترس باشد
اگر چه بیمارستانها ممكن است انواع مختلف پانسمان موجود باشند،اما گاهی تمامی آنها در سطح جامعه برای عموم در دسترس نیستند.بیمار باید بعد از ترخیص از بیمارستان نیز درمان را ادامه دهد.
The 'ideal' dressing that meets all these requirements for every wound type does not yet exist . However, in the vast array of dressings available, it is quite possible to select one for achieving one's goal.
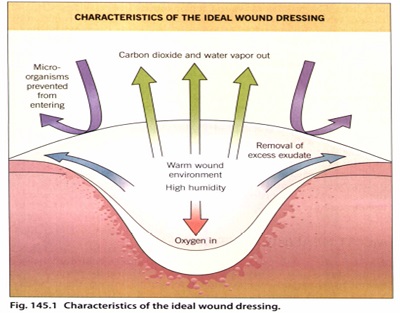
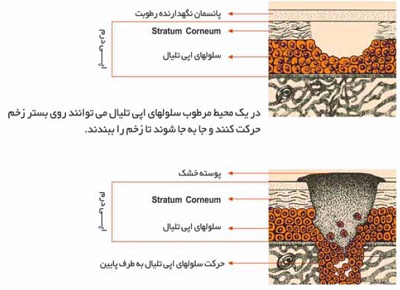
MOIST HEALING ENVIRONMENT
A scab or eschar forms as a result of the drying of a wound, particularly of the superficial dermis which itself become integrated into the scab.
The studies of Winter and Scales illustrated that uncovered, air-dried wounds developed thicker scabs and re-epithelialized at a slower rate. This slower rate has been attributed to the requirement of the regenerating epidermis to migrate deeper below the dry fibrous tissue to a region of moisture where live cells survive. therefore, the thicker the scab, the deeper the migration.
Many endogenous factors that are critical to wound healing (e.g.fibrin degradation products, platelet-derived growth factor) are found in fluid from occluded (or dressed) acute cutaneous wounds and may be more available in a moist environmcnt .
Another possible value of a moist wound environment may be its ability to confer an electrical gradient between the wound and normal skin. That is, upon injury of skin, an internal battery and a current flow are created until drying of the wound occurs. It is thought that in maintaining moisture, this electrical gradient may promote epidermai cell migration between the wound and surrounding skin.
THE ROLE OF OXYGEN
the oxygen requirement is initially low during the early wound repair stages. Hypoxia has been shownto
upregulate proliferation and production of TGF by dermal fibroblasts; TGF is known to stimulate production of extracellular matrix molecules.
Keratinocytes migrate better along collagen and fibronectin, and low oxygen levels also promote angiogenesis in the acute wound.
The use of a semipermeable dressing can provide the appropriate oxygen tension for wound repair to proceed quickly.
مراحل ترمیم زخم
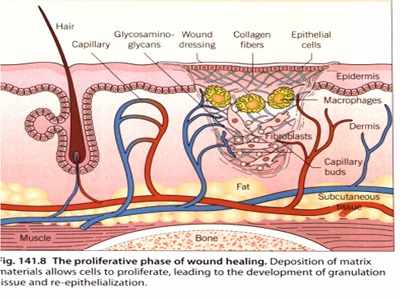
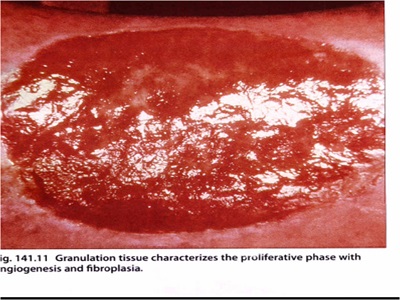
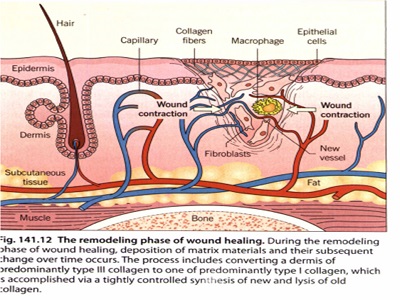
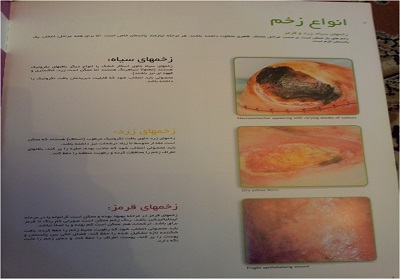
تقسیم بندی انواع پانسمان
Bolognia
-Traditional
-occlusive
Foams/Films/ Hydrocolloids/Alginates/ Hydrogels
Absorptive:
Gauze / Foams
Occlusive
Nonbiologic:
Films/ Hydrocolloids/Alginates/ Hydrogels
Biologic :
Homograft / Xenograft / Amnion / Skin substitutes
TRADITIONAL WOUND DRESSINGS
Traditional or conventional dressings have commonly been made of natural, synthetic or partially synthetic materials. such as cotton, silk, linen or cellulose-based substances
The basic cotton gauze dressing in use today is frequently composed of cotton plus cellulose acetate (added for increased absorbency).
Petrolatum and other ointments such as soft paraffin wax, with or without antibacterials such as povidone-iodin;sulfadiazine, bismuthtribromophenate (Xeroform@), framycetin and chlorhexidine,are examples of impregnated substances;
These medicated dressings are often composites of rayon, nylon or gauze, and are used for malodorous wounds such as chronic ulcers. Activated charcoal cloth (with or without antibacterial silver salt) is also used for exudate absorption plus odor control.
These types of dressings are placed directly against the wound bed and have the advantage :
the ability to mold into the depression of deeper wounds fo the purpose of filling dead space and providing absorption.
relatively inexpensiv
mesh gauze is excellent at wicking exudate away from a wound, although it loses effectiveness when saturated e and readily available,
gauze, lint and cotton dressings, other simple modified absorbent pads covered with a perforated plastic film to prevent adhering to a wound (such products include Melolin™, Cutilin™ and Telfa®) are used both as primary and secondary dressings. They are used in minor and low exudating wounds.
Exudry™ and Mesorb® are examples of products with a highly absorbent pad and a non-stick, nonshear surface. They can be used as a secondary dressing over moderate to highly exudating wounds and over hydrocolloid paste, cadexomer iodine, alginate.
The non-absorbent passive dressings such as paraffin gauze (tulle) dressings
A layered dressing in three parts :
(I) the contact or interface layer, which is usually a non-adherent, fluid permeable
(2) the absorbent layer, usually a cotton pad, gauze or other such material, help the dressing mold to the shape of the wound;
(3) the outer layer or wrap, often tape or other banding material for retention of the underlying layers. Of note, each layer should be placed in close approximation to the one before it, with no gaps or air pockets, and should increase in size and degree of overlap, from wound bed to outermost layer.
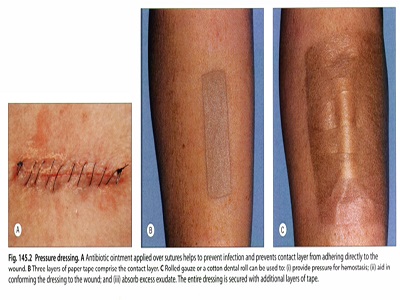
usage
Postsurgical skin wound defects healing by first intention are those that are clean, free of debris and sutured by aseptic technique. After suture removal, the use external splinting tape (such as Steri-StripsTM) supports the tissues, enabling favorable collagen remodeling that may limit scar formation and hypertrophy.
Second intention healing is often employed following many cutaneous surgical techniques such as punch biopsy, cryosurgery, laser surgery, excision and curettage.
A semiocclusive dressing is the treatment of choice, topical ointment directly on the wound. Monitoring of the wound as for signs of infection prevention of drying of the wound bed until re-epithelialization
These types of dressings are placed directly against the wound bed and have the advantage :
the ability to mold into the depression of deeper wounds fo the purpose of filling dead space and providing absorption.
relatively inexpensive.
The disadvantage of this type of dressing is the potential for maceration of the wound and surrounding skin should the dressing remain in place for an extended period of time.
they do require frequent replacement, which is time-consuming for the medical staff and the patient and is potentially costly in healthcare personnel time.
TOPICAL ANTIMICROBIAL AGENTS
The usefulness of topical antimicrobial agents for cutaneous wounds is a matter of debate.
It is thought by some that a clean wound created with good aseptic technique does not require a topical antimicrobial, as long as the wound is well cared for in the postsurgical period.
a group of patients with long standing, non-healing leg ulcers. There are some clinicians who believe that one of the reasons for non-healing of these wounds is the presence of large numbers of colonised bacteria, especially in those patients who may be malnourished, diabetic or immuno-compromise
nanocrystalline silver comes in a variety of dressing types (e.g. ActicoaeM, Actisorb@ Silver, Contreet Foam,Contreet Hydrocolloid and SilverlonTM)
-broad-spectrum antibiotic activity against both Gram-positive (including methicillinresistan Staphylococcus aureus [MRSAJ) and Gram-negative pathogens These dressings can release antibacterial of silver for 3 to 7 days.Silver ions kill microorganisms
no documented evidence of either bacterial resistance or cytotoxicity from these dressings Silver
also appear to decrease the levels of matrix metalloproteinases that are upregulated in non-healing, chronic woundssepsis and bacteremia as well as shorten hospitalization time..
Although povidone-iodine, another commonly used antiseptic, can inhibit wound healing, newer formulations - such as cadexomer-iodine polymer, which slowlyreleases iodine from dextran beads - have not demonstrated toxic effects on keratinocytes. Studies have shown a significant decrease in ulcer size with cadexomer-iodine when comparedto hydrocolloid and paraffin gauze dressings.
Recommended
for exudative wounds such as leg ulcers, a layer of beads is applied to the wound and covered with a pad or other suitable secondary dressing.
Changes three times per week as the healing progresses.
Because the iodine is absorbed, caution must be taken when using these dressings in patients with a history of thyroid disease. They should not be used in young children, pregnant and lactating women, or patients with a known or suspected iodine sensitivity, Hashimoto's thyroiditis or a history of Graves disease.
Cadexomer-iodine is also found in multiple types of occlusive dressings, including hydrocolloids (e.g. Iodosorb@) and hydrogels (e.g. Iodoflex@
OCCLUSIVE DRESSINGS
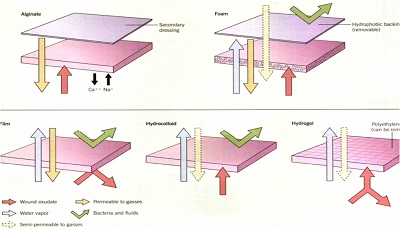
Polymer Films
Polymer films are thin, elastic, transparent sheets of polyurethane or other synthetic semipermeable, self-adhesive polymer dressing.
are waterproof.
are gas vapour permeable. allowing for the exchange of oxygen, carbon dioxide and water vapor proteins and water of wound fluids and bacteria are prevented from moving across the dressing.
are flexible
protect from shear, friction, chemicals and microbes are transparent
spread tension forces.
Uses
cover intravenous sites and sites of partial-thickness wounds such as skin tears, superficial decubitus ulcers, split-thickness graft donor sites, burns ,laser wounds, dermabrasion, and Mohs surgery defect sites.
They are also useful for holding grafts in place for venous ulcer treatment. As the dressing allows for the visual monitoring of the wound, it may be used to hold a graft in place until engraftment.
An additional piece of film may then be used to secure the graft instead of using sutures
Examples
BioclusiveM (Johnson & Johnson); Opsiteı (Smith & Nephew); Polyskin@ II (Kendall); ProCyte Transparent Film Dressing (Bard Medical); Silon-TSR@ (Bio Med Sciences); and TEGADERMı.
There are several film dressings, however, that have no adhesive at all or have an adhesive-free zone. Examples of such dressings are Blister
Film@ (which is composed of polyester rather than polyurethane! And Omniderm@.
Advantages/disadvantages
its translucency, thereby allowing direct visualization of the wound without removal.
its permeability to water vapor; and its tendency to reduce postoperative pain. It is also thought to enhance re-epithelialization of graft donor sites, with a reported increase in healing rates of 25_45%
One disadvantage of film dressings is that they are difficult to place properly
As the film usually only adheres to intact skin, a 1-2 em application margin is recommended.
Films may also adhere to the wound as drying progresses, thereby risking disruption or stripping of the newly formed epithelium, which is not yet tightly bound to the underlying dermal layer
It is possible also to traumatize newly grafted skin tissue during dressing changes. For these reasons, it is advised to allow the film dressing to remain in place until it spontaneously falls off, which can occur after 1-2 weeks
Another disadvantage of film dressings is that they are nonabsorbent; therefore, wound fluid can accumulate under the dressing layer, especially with highly exudative wounds. not adversely affect wound healing; significant bactericidal activity. A 27- or 30-gauge needle can be used to puncture the film for the purpose of aspirating the exudate, The elevated bacterial counts, however, have not correlated with an increased rate of infection.
Polymer Foams
Polymer foam dressings are semi-occlusive, bilaminate, and polyurethane- or silicone-based. They consist of a hydrophilic foam with a hydrophobic backing, which then prevents leakage, provides a barrier against bacterial penetration, and provides the moist environment.
.The outer layer is a semipermeable, non-absorbent membrane composed of polyurethane,polyester, silicone surrounded by a polyoxyethylene glycol foam.
Uses
wounds from Mohs surgery, surgical incisions,dermabrasion and burns; chronic wounds such as diabetic ulcers, venous ulcers and pressure ulcers (stages I, II and III); and deep cavity wounds refractory to other methods
Examples
Adherent and non-adherent: Allevynı (Smith & Nephewb Biatainı (Coloplastb Curafoamı and Hydrosorb@(Kendall); Flexzan@(Bertek);Lyofoam@(Convatecb Restonı (3Mb and Sof-Foamı (Johnson & Johnson).
Advantages/disadvantages
Although very absorbent, there is a limit to the amount of wound exudate this type of dressing can absorb.
Therefore, it should be changed every 1-3 days.
The permeability of foams to both gas and water vapor makes them suitable for mild to moderately exudative wounds, although there are brands designed for heavily exudative wounds.
The disadvantages of foam dressings are the inability to use them with dry wounds, their opacity, which prevents visual monitoring of the wound, and the need for frequent changing.

Hydrogels
Hydrogels are a group of complex organic polymers having a high water content ranging between 30 to 96 percent.
consists of a cross-linked hydrophilic polymer network composed of polyvinyl alcohol, polyacrylamides, polyethylene oxide or polyvinyl pyrrolidone; it is produced as sheets, amorphous gels(pre-mixed or dry), or as impregnated dressings
Hydrogels are semitransparent , have a high absorptive capacity (between 100% and 200% of their volume), and are able to maintain a moist wound environment. . This broad class of polymers swells extensively in water, but does not dissolve in water.
Hydrogels are described as semi-adherent or completely non-adherent, depending upon the subtype, and therefore require a secondary dressing to hold them in place.
. sheet form of a hydrogel dressing is constructed by sandwiching the hydrophilic polymer between two removable thin sheets of polyethylene film.
For application to the wound, the film on the contact side is removed, leaving the outer film in place. With this mode of application, the dressing is semipermeable to gases (including oxygen) and water vapor. If the outer film is also removed, then the dressing becomes permeable to fluid as well; as a result, exudate can pass to a secondary gauze dressing
Uses
Hydrogels are particularly useful with dermabrasion, laser resurfacing,chemical peels, superficial thermal burns, ulcers, surgical wounds such as graft donor sites, and partial-thickness wounds in which an interval of light to moderate exudate production is likely.
hydrogel dressings are indicated for dry to minimally exudative wounds with or without depth, and are a good choice for painful wounds because hydrogel dressings do not adhere to the base of the wound. International pressure ulcer guidelines recommend considering the use of hydrogels for dry to minimally exudative pressure ulcers (NPUAP-EPUAP, 2009). Hydrogel dressings are formulated as sheets or amorphous gel (see
Examples
Hydrogel sheets: CarraDresı Clear Hydrogel Sheet, CarraSorbı Freeze
Dried Gel (CarrinhTtOn Labs); Nu-GeFM (Johnson &. Johnson); and
Vigilonı (Bard Medical!.
Amorphous gels: Biolex@ Wound Gel (Bard); CarraSynTM Hydrogel
(Carrington); GRX Wound Gel (Geritrex Corp.); Intrasiteı Gel (Smith
&.Nephew); and Tegagelı Hydrogel Wound Filler (3M Health Care).
Impregnated dressings: DermaGauzeı (DermaRite) and TegagelıWound Filler with gauze (3M Health Care).
Advantages/disadvantages
reduction in postoperative pain and inflammation.
accelerate the rate of wound healing when compared to traditional Telfa@ and gauze dressings.
soothing and actually lower the temperature of cutaneous wounds, a cooling effect that can be augmented by refrigerating the dressing prior to application.
Disadvantage
allow bacterial growth at the wounds electively permit Gram-negative organisms to proliferate. For this reason, antibiotic ointment is often applied prior to the application of the dressing. site studies have not shown an increase in infection rate. more frequent dressing changes than is seen with other occlusive dressings.

Alginates
Alginate dressings are made from a natural, complex polysaccharide derived from various types of algae or kelp .
Upon application of the dressing, a reverse ion exchange occurs between the calcium within the alginate fibers and the sodium from blood or the wound exudate. This results in the formation of a soluble sodium alginate gel that fills and completely covers the wound in a non-adherent manner, providing a moist wound healing environment. release of calcium augments the clotting cascade,
producing the hemostatic advantage.
Uses
To secure an alginate dressing, a secondary dressing is required. A dressing change is indicated when the dressing has been in place for several days or when exudate soaks through to the secondary dressinig.
highly exudative wounds. Full-thickness burns and surgical wounds, split-thickness graft donor sites, Mohs surgery defects, and refractory decubiti and chronic ulcers
used on dry wounds as well, but pre-application moistening with saline is necessary and the dressing should be changed on a daily basis to limit desiccation.
Examples
Algiderm@ (Bard Medical); AlgiSiteM (Smith &.Nephew United); FibracolQ9 Plus, a collagen-alginate complex dressing (Johnson &. Johnson); Kaltostat@ (ConvaTec); and Sorbsan@ (Bertek


Advantages
removed by saline irrigation, which allows for less painful dressing changes.
encourage wound healing highly absorbent, which allows them to remain in place at the wound site for several days at a time, thereby
minimizing dressing changes.
disadvantage
Removal of the dressing in a dried state can reinjure the wound.
yellow-brown appearance, which can be confused with purulent discharge. Therefore, careful wound monitoring for signs of infection is indicated. Have an unpleasant odorremoval of the secondary
Hydrocolloids
Colloids are characterized by the strength of attraction of the particles to the continuous medium and to the proportion of water within that medium. Since cells and tissues throughout the body are comprised of colloids, it seems reasonable that the creation of an environment similar to that found on cell surfaces would aid in the healing process
Sheets with an inner adhesive layer consisting of a hydrophilic colloid base that is a mixture of pectin, karaya, guar or carboxymethyl cellulose and an adhesive containing polyisobutylene, styrene isoprene or ethylene vinyl acetate
The outer layer is composed of a thin semipermeable material such as polyurethane.
A gel is formed in the presence of wound exudate, and as a unit, the dressing is semipermeable to water vapor and gases27
Another type of hydrocolloid dressing is a synthetic, non-adherent, high-density plastic woven polymer (e.g. N-Terface@). Fluid is able to flow through this matrix to be absorbed by an overlying dressing without adherence to the new epithelial surface
Uses
Hydrocolloids are used for the treatment of burns, partial-thickness and dermabrasion wounds, traumatic wounds such as lacerations and abrasions, surgical wounds such as donor graft sites and excisions, chronic ulcers, bullous disorders such as epidermolysis bullosa, and refractory inflammatory diseases such as lichen simplex chronicus and psoriasis.
are available that enhance adherence to anatomically challenging sites, such as the sacrum, heel, knee, and elbow
Advantages
When in sheet form, these dressings can be cut and conformed to the shape of the wound.
They also adhere directly, are waterproof, and therefore do not require a secondary dressing. They also have a cushioning or pressure-relieving effect (especially at bony sites),
results in autolytic debridement that can be washed away with saline irrigation of the wound bed28stimulate angiogenesis and increase the rate of healing by as much as 40% when compared to open air controlsdo
not require as many dressing changes
disadvantage
maceration of the wound's surrounding skin is possible. Leakage of excessive tissue exudate can also occuthere is the possibility of the growth of excessive granulation tissue
yellow-brown, thick, foul-smelling gel resembling purulent discharge.
bacterial proliferation and colonization
within the moist environment underneath the dressing, but have not shown increased rates of infection as a result. Some investigators,
in fact, have reported a decrease in the growth of Pseudomonas.
Composites
The designs of occlusive dressings are constantly changing, with the goal of improved and simplified care for a greater range of wounds.
Several new types of composite dressings, which combine two or more types of semi-occlusive dressings into one product .They have three components: (1) a semi- or non-adherent layer that contacts the wound (like a hydrogel, hydrocolloid, foam or alginate); (2) an absorptive layer; and (3) an outer layer (like a film with an adhesive border).
Tissue-Engineered Skin Equivalents
Advances in tissue culture techniques for keratinocytes and fibroblasts,as well as new methods that allow the generation of large quantities of these cells, are rapidly being incorporated into a new group of wound dressings.
For chronic ulcers that are resistant to
healing, skin equivalents can be used.
advantage is that painful,often slow-to-heal donor sites do not have to be created
There are three types of skin substitutes: epidermal grafts, dermal replacements, and composite g'rafts(with both an epidermal and dermal component).
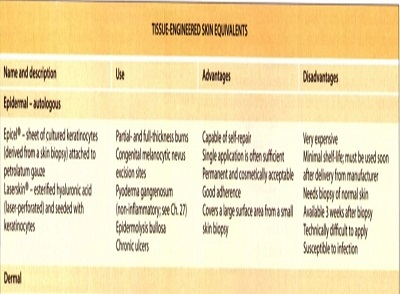
Epidermal grafts
They have been used successfully in a variety of clinical situations, including burns, chronic ulcers, vitiligo and postsurgical wounds. approved for use in partial- and full-thicknes burns as well as following removal of congenital melanocytic nevi
A skin biopsy is first obtained from the patient and then the autologous keratinocytes are co-cultured with irradiated murine fibroblasts that serve as a feeder layer. A sheet of keratinocytes two to eight cells thick forms and is attached to petroleum gauze. The graft must be sutured into place and the gauze backing is removed after 1 week, when the keratinocytes have attachedto the wound.*
disadvantages are the short shelflife, the expense, and the 3-week interval required for the culture of the keratinocytes.
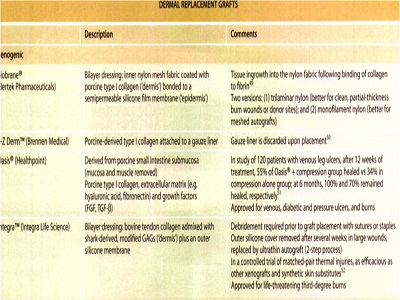
Dermal replacements
two types of dermal replacements: allogenic and xenogenic.
Neither of these is permanent: DNA analysis of wounds after the application of non-autologous skin substitutes shows complete disappearance of grafted cells after 2 months. The goal of these dermal grafts is to provide a temporary biologic dressing in order to stimulate the healing process. The major component collagen, and occasionally there are additions such as extracellular matrix (fibroblasts and basement membrane material.
Xenogenic
The advantages of these products are their ability to affect hemostasis and to provide immediate closure, cosmetically acceptable scars . safety from potential human pathogen transmission due to their animal origin, and adequate shelf-life.
disadvantages ineffective over exposed bone, and there is the potential for infection if not cared for adequately. Bovine allerg'Y is also a possibility. Biobrane@ may adhere to the wound so tightly that it could damage newly developed epithelium and inhibit wound contraction.


Allogenic
The advantages of these products are their ability to provide immediate closure, cosmetically acceptable scars with second intention healing, and adequate shelf-life, facilitating off-the-shelf access.
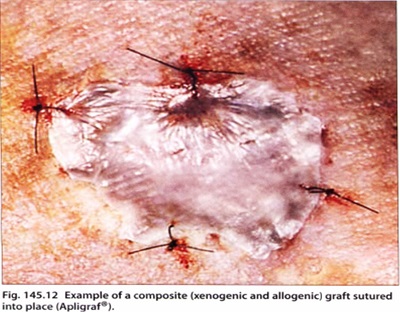
Composite grafts
bilayered human skin equivalent derivedfrom human neonatal foreskin. It contains living human keratinocytes within an epidermal layer and a dermal layer. bovine collagen type I gel seeded with fibroblasts.
Apligraf@ has been used to treat both acute and chronic wounds that fail to heal by standard means.
a single application may be sufficient to initiate a satisfactory healing response
It is currently approved by the (FDA) for venous and diabetic ulcers, but has also been shown to be useful in epidermolysis bullosa, pyoderma gangrenosum,burns and surgical excisions.
Nevertheless, these composite grafts may be justified in certain situations when adequate skin grafts cannot be obtained from the patient.
SPECIAL CONSIDERATIONS DRESSINGS FOR LEG ULCERS
venous, arterial and neuropathic; less commonly, they are due to inflammation, infection, trauma or malignancy
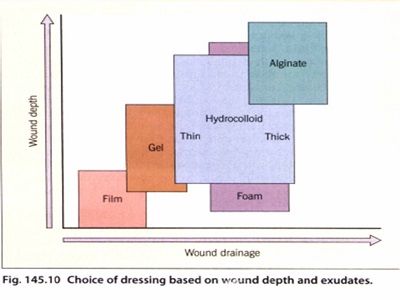
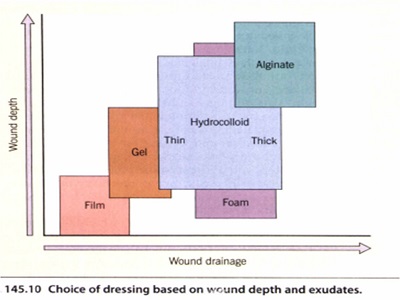
The five major categories of occlusive dressings are simplified in. Only a few comparative studies of dressings have been conducted,
Compression is considered to be of utmost importance in the care of venous ulcers.
the pressure of surface veins, thereby decreasing macromolecule and fluid extravasation. Compression is also said to promote fibrinolysis and ultimately improve cutaneous blood flow once edema has been mechanically reduced.
Compression Stockings
for arthritic and/or elderly patients, application can be difficult.
Compression Bandages
Unna boot:
Applied in a semi-rigid state, it confers (semi-rigid) compression along with the advantages of a moisture-retaining occlusive dressing Correct application of the Unna boot prevents excessive or abnormal pressure to the limb, compromised circulation, skin breakdown, additional ulcer formation or further limb deterioration.
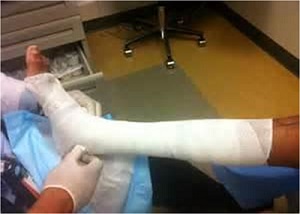
Multilayer Compression Bandages
The four layered system can remain in place for up to 1 week and has the advantage of providing absorption as well as evenly distributed pressure throughout the leg. the first of which is an orthopedic wool layer applied loosely in spiral fashion. It absorbs exudate as well as redistributes pressure around the ankle. The second layer is a crepe bandage, used to smooth the wool layer and
to increase absorbency. The third layer is a light elastic compression followed by the fourth and last layer, which is the outer, elastic, cohesive bandage used to secure all layers in place.
Types of Paste Dressings
are used to treat the chronic dermatitis associated with leg ulcers,by creating an absorptive, protective, contact layer against the wound surface and surrounding skin.
These open-weave, cotton bandages are impregnated with zinc paste, calamine or ichthammol, all of whichhave a soothing action on irritated skin; The skin of leg ulcer patients is often easily sensitized to topical medications. Therefore, it becomes important in such patients to perform patch testing prior to bandaging if sensitization is suspected.

Growth Factors, Heat and Enzymes
The use of infrared radiant heat bandages has been effective in the laboratory as well as in small trials in healing leg ulcers which are refractory to other methods and in ulcers
Enzyme agents
Debridement can be accomplished by the following mechanisms:
surgically; mechanically via irrigation, wet-to-dry dressings;
autolytically via compression bandaging and the use of occlusive dressings such as hydrocolloids and alginates; chemically
Collagenase (Santyl@) is reported to be effective inthe removal of devitalized tissue and to contribute to the formation of granulation tissue and ulcer epithelialization, without attacking healthy or new granulation tissue.
enzymatic debridement is slower than mechanical but is frequently used for initial debridement when anticoagulant therapy The length of time required to achiev debridement may range from several days to weeks. Unlike autolysis, enzymes may also be used to debride a wound with significant bacterial infection (Ramundo and Gray, 2008).
Papain (e.g. Accuzyme@, Panafil@)
is harmless to viable tissue, while functioning as a potent digestive agent to non-viable tissue.
Its action, however, requires the presence of an activator such as urea with which it is processed, since papain alone is relatively ineffective;
Trypsin
(e.g. Granulex@, Balsa-Derm@) is processed with balsam of Peru (which is an effective capillary bed stimulant and mild bactericide) and castor oil, which is used to reduce premature epithelial desiccation and cornification, as well as pain
Fly larvae have been used for centuries to biosurgically debride chronic ulcers. Today, the most commonly used maggots are those of the green bottle fly Lucilia sericata.
only digest necrotic tissue, leaving healthy tissue exposed. This digestion is accomplished though collagenases and trypsin-like enzymes with minimal destruction of normal tissue.
They also have antimicrobial effects via secretion of antibacterial compounds (e.g. phenylacetic acid, phenylacetaldehyde) and the ingestion and subsequent killing of Grampositive (including MRSA) and, to a lesser extent, Gram-negative bacteria.
considered a last resort option when the patient is not a surgical candidate and the wound has not responded to conventional methods of debridement. A review of evidence concluded that maggot therapy offers no more overall effectiveness than other methods of debridement (Gray, 2008).
Biosurgical therapy should not be used with wounds that are poorly perfused, require frequent inspection, or have exposed blood vessels, necrotic bone, or limb-threatening infections (NPUAP-EPUAP, 2009
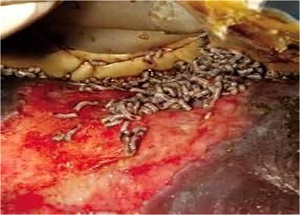
SPECIAL CONSIDERATIONS
Laser Resurfacing
it does require a prolonged and often painful recovery period for the patient. The primary goal after laser resurfacingis total and uniform re-epithelialization. In the absence of infection and with the use of occlusive dressings, this is possible within 5-7 days.
An open dressing is defined as the application of an ointment or cream directly to the skin wound without an overlying textile dressing
method requires the commitment of the patient to maintain adequate coverage of the wound by frequent, scheduled ointment reapplication
an open dressing does result in less bacterial colonization than closed dressings,it allows for easy wound monitoring, and it is effective and less expensive than the closed wound care technique.
Antibiotic ointment applied to facial wounds of less than48 hours has been reported to significantly increase the rate of contact dermatitis and is therefore not recommended.
used for open dressings are: petrolatum, Aquaphor@ (Beiersdorf-Jobst),Complex CU3@ ointment (Procyte), and Elta@ Creme (Swiss-American Products)
Closed dressings are found among any of four main occlusive dressing categories .
(Flexzan@), hydrogel (2nd Skin@), polymer film (Silon-TSR@), and hydrocolloid
The immediate placement of a closed dressing after surgery was followed by return of the patient on postoperative days 1 and 2, at which point the wound was cleaned and the entire dressing was changed. There was no perceptible delay or interference in re-epithelialization by changing the dressing at this point. The replacement dressing remained in place until postoperative day 5, at which time the closed dressing was removed and the patient was converted to open dressing alone5
Absorbency without saturation, placement stability, ease of use, patient comfort, and minimal adherence to the newly developing epithelium are the desirable characteristics of a closed dressing for laser resurfacing. when compared to open dressing techniques, closed dressings overall resulted in faster re-epithelialization with improved patient comfort.
HONEY
Indian J Plast Surg. 2012 May-Aug; 45(2): 418–424. Wound care with traditional, complementary and alternative medicine
Composition and properties of honey
Honey has approximately 40% fructose, 30% glucose, 5% sucrose and 20% water. It also contains several amino acids, antioxidants, vitamins, minerals, glucose oxidase, which produces hydrogen peroxide, and gluconic acid, which gives the honey an acidic pH of 3.2-4.5. Since Clostridium botulinum is able to survive in honey, there is a risk of botulism or gangrene. Hence, it is advocated to sterilize the honey using gamma irradiation .
Honey has a unique property of delivering a moist wound healing environment due to its highly viscous nature.Due to the hyperosmolarity of honey, it is able to absorb the exudates from the wound and enable the wound to heal in a moist environment. Honey has anti-bacterial, anti- inflammatory and anti-fungal properties
Honey has been documented to increase wound contraction and wound epithelialization in animal and human studies
Honey has the inherent capability to increase the formation of granulation tissue, stimulate tissue growth, and reduce edema, inflammation and the synthesis of collagen. Honey is able to reduce the incidence of post-operative adhesions in intra-abdominal tissues.Honey has the potential to deodorize the wound and also reduce pain
The major medical-grade honeys approved for clinical application are Manuka and Revamil®. Manuka honey which is produced from the manuka bush (Leptospermum scoparium) originates from New Zealand and Australia and has been widely tested worldwide. However, almost every sub continent has individual sources of honey and has reported successful results in wound therapy.
CURRENT SCIENTIFIC EVIDENCE ON THE USE OF HONEY IN WOUND MANAGEMENT
Numerous clinical trials have been conducted to test the efficacy of honey in wound management. The focus of this article will be on human trials. A total of 20 clinical trials have been conducted on acute and chronic wounds.
In acute wounds, a total of 15 clinical trials have been conducted using honey. Acute wounds can be subdivided into acute burn wounds and acute wounds not caused by burns.
Acute burn woundsA total of 11 clinical trials.
Subrahmanyam et al. conducted two trials (n = 154) on superficial burn wounds. In both the trials, the control group used silver sulfadiazine (SSD) and honey-treated wounds had better healing rates.Gupta et al. conducted a trial (n = 108) on superficial and partial thickness burn wounds, comparing honey with SSD. They also noted better healing rates with the honey group (18.1 vs. 32.6 days).Six trials (n = 1326) were conducted on partial thickness burn wounds. Five of the six trials were conducted by the same researcher. In all the six trials conducted on partial thickness burn wounds, honey showed better healing .
two trials on mixed partial thickness and full thickness burn wounds. In the first study (n = 50), they compared honey dressing with tangential excision and skin grafting. The skin grafting group showed better healing patterns compared to the honey-treated group (18.4 vs. 32 days).
In the second study, honey was compared with SSD in 100 patients with less than 40% total body surface area (TBSA) of burns. The healing rates were better with the honey-treated group (15.4 vs. 17.2 days
From these selected clinical trials, there seems to be a trend in favour of honey in the management of superficial and partial thickness burn wounds, but strong evidence is lacking. In the management of full thickness burn wounds, honey does not seem to improve healing as only two studies have been conducted. Hence, more clinical trials are needed to substantiate the evidence of the use of honey in superficial and partial thickness burn wounds
Non-burn acute wounds
Four clinical trials have been conducted
Two studies on surgical wounds created following partial or total toe nail avulsions. In the first study (n = 51), Marshal et al. failed to report better healing rates with honey compared to iodine (33.4 vs. 25.4 daysIn the second study (n = 100), McIntosh et al. also did not achieve better healing rates with honey compared to paraffin gauze dressing (40.3 vs. 39.98 days). [
There was only one trial (n = 87) conducted on laceration or shallow abrasion wounds in 2006 by Ingle et al. In this study, the authors compared honey with hydrogel dressing in abrasion wounds between 10 and 100 cm2. There was no difference in the healing rates (16.48 vs. 16.88 days.
there is insufficient evidence to support the use of honey in acute wounds such as abrasions, lacerations or toe nail avulsions. A trial (n = 75) was conducted by Farrah et al. on split skin graft donor sites using honey hydrogel and plain hydrogel. The honey hydrogel group showed better healing rates at days 10 and 15.
CHRONIC WOUND
Based on these limited studies, it is inappropriate to conclude that honey has a beneficial role in chronic wounds. However, larger randomized clinical trials are advocated to further substantiate the usefulness of honey
آقاي ساسان سلام
اگر بيماري در حال پيشرفت باشد،نقاط جديد بدن كه تازه دارند رنگدانه از دست مي دهند ولي هنوز روند پيشرفت ادامه دارد،خيلي سفيد نيستند،در خصوص سوال دوم،چنين آمپول و درماني وجود ندارد،بهترين درمان براي شما نور درماني هست .
زمان بهترین و ارزشمندترین هدیه ای است كه می توان به كسی ارزانی داشت.هنگامی كه برای كسی وقت می گذاریم، قسمتی از زندگی خود را به او میدهیم كه باز پس گرفته نمی شود . باعث خوشحالی و افتخار من است كه برای عزیزی مثل شما وقت می گذارم و امیدوارم كه با راهنماییهای اساتید این رشته واظهار نظر شما عزیزان این سایت آموزشی پر بارتر گردد.